What Is the Best Lewis Structure for Cs2
What is the formal charge on the central Cl atom. Who are the experts.
Cs2 Lewis Structure How To Discuss
The core atom is carbon which is flanked by two sulfur atoms.
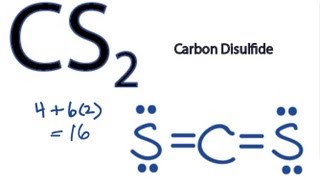
. To answer the questions interpret the following Lewis diagram for AsO2-1. Place the following in order of increasing dipole moment. Double lines 2 Ss 1 C 4 dots total.
Carbon is the least electronegative atom and goes in the center of this structure. Carbon is the least electronegative atom and goes in the center of this structure. The best place to start when trying to figure out a molecules geometry is its Lewis structure.
Best Lewis Structure The Lewis structure that is closest to your structure is determined. Is there something else I should know for the purpose of answering a question like this on my test. Carbon is the least electronegative atom and goes in the center of this structure.
Prev 12 of 16. Choose the best Lewis structure for ICl5. The impure carbon disulfide that is usually used in most industrial processes is a yellowish liquid with an unpleasant odor like that of rotting radishes.
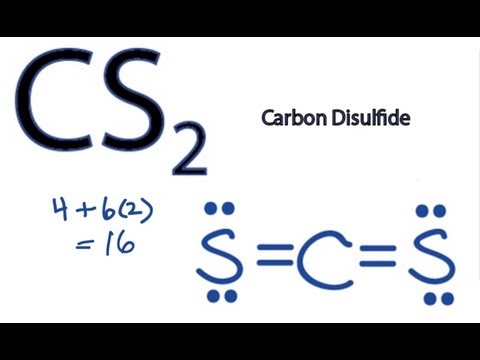
There are 16 valence electrons available for the Lewis structure for CS 2. Please note that your structure cant be well described by a single Lewis structure because of extensive delocalization. Chemistry questions and answers.
If playback doesnt begin shortly try. Saved Identify the correct Lewis structure for CS2. A valid Lewis structure of CS3 cannot be drawn.
The sulfur atom is required two electrons to complete the octet of sulfur atoms. Carbon disulfide evaporates at room temperature and the vapor is more. Draw the best Lewis structure for CS2 that satisfies the octet rule.
Choose the best Lewis structure for OCl2. The hybridization of the atoms in this idealized Lewis structure is given in the table below. CS2 is an abbreviated form of Carbon Disulphide.
Hybridization in the Best Lewis. This species has its three atoms bonded sequentially in the following fashion. Sulfur contains six outermost valence electrons which means it contains six electrons in its outermost shell whereas carbon has four outermost electrons.
View HW 81docx from CHEM 1123 at University of Texas San Antonio. What is the electron-pair geometry around the central atomEle. Double bonds act as one electron pair to help determine electron-pair geometries of molecules according to VESPR.
The central carbon atom will form double bonds with the two sulfur atoms. The electron-pair geometry of CS2 is linear because the Lewis structure is SCS. Use the following Lewis diagram for 1-propanol to answer the questionsRemember that geometry refers.
We review their content and use your feedback to keep the quality high. The electron-pair geometry of CS2 is linear because the Lewis structure is SCS. My answer was that a valid structure cannot be drawn because CS3 displays the property of resonance.
The Lewis structure for CS2 requires you have double bonds between the Carbon C and Sulfur atoms in order to fill the octet of Carbon. CS2 Lewis Structure Hybridization Polarity and Molecular Shape. Double bonds act as one electron pair to help determine electron-pair geometries of molecules according to VESPR.
Clearly indicate bonds and non-bonding electrons. CS 2 is named Carbon Disulfide. Choose the best Lewis structure for SO4 2-Draw the best Lewis structure for Cl3-.
The lewis structure for CS2 is. There are 16 valence electrons for the CS2 Lewis structure. For the central arsenic.
The Lewis structure for CS2 requires you have double bonds between the Carbon C and Sulfur atoms in order to fill the octet of Carbon. Experts are tested by Chegg as specialists in their subject area. Carbon disulfide CS2 will have a total of 16 valence electrons 4 from the carbon atom and 6 from each of the two sulfur atoms.
This molecule has two Sulphur atoms and one Carbon atom. To understand the hybridization molecular geometry and the polarity of this molecule it is essential to under its Lewis structure. The Lewis structure for CS2 requires you have double bonds between the Carbon C and Sulfur atoms in.
The formal charge on the bromine atom is BrO3 drawn with three single bonds is. See the Big List of Lewis Structures. View the full answer.
100 1 rating Number of valence e. Am I correct in this assumption. Is CS2 polar or nonpolar.
The Lewis structure for CS2 is shown. Pure carbon disulfide is a colorless liquid with a pleasant odor that is like the smell of chloroform. BrF3 CS2 SiF4 SO3.
Drawing the Lewis Structure for CS2. There are 16 valence electrons for the CS2 Lewis structure. Predict the geometry around the central atom in SO42-.
The Lewis structure for CS2 is.

Cs2 Lewis Structure Carbon Disulfide Youtube

Cs2 Lewis Structure How To Draw The Lewis Structure For Cs2 Youtube
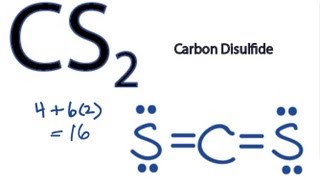
Cs2 Lewis Structure How To Draw The Lewis Structure For Cs2 Youtube
No comments for "What Is the Best Lewis Structure for Cs2"
Post a Comment